| Name |
Japan CRO Association |
| Location |
504 Nihonbashi Life Science Bldg., 2-3-11
Nihonbashi Hon-cho, Chuo-ku, Tokyo Japan 103-0023 |
| Established |
Sept. 1994 |
| Number of Members |
49 companies
(13 regular members, 36 associate members) |
| Chairman |
Hisashi UEMATSU [Japan CRO Association] |
| Vice Chairman |
- Toru FUJIEDA [CMIC HOLDINGS Co., LTD]
|
| Director/Auditor |
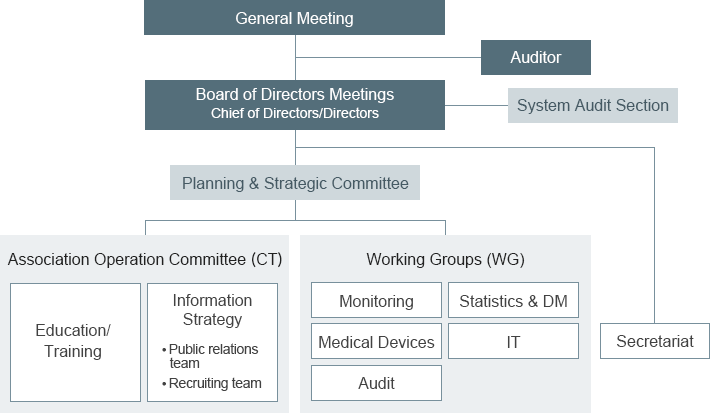
- Directors
- Hitoshi KAMIYA [A2 Healthcare Corporation]
- Yuichi YAHAGI [INTAGE Healthcare Inc.]
- Hideyasu MATSUDA [IQVIA Services Japan G.K.]
- Fumiaki HAMANO [Fortrea Japan K.K.]
- Shigehiro MIKI [PAREXEL International Inc.]
- Auditors
- Masayuki HAYASHI [DOT WORLD CO., LTD.]
- Masashi IWASAKI [intellim Corporation]
|
| Special Advisors |
Kazuo NAKAMURA [CMIC HOLDINGS Co., Ltd.] |
| Business activities |
Japan CRO Association (as JCROA hereinafter) is composed by CRO (Contract Research Organization) member companies in Japan that contract the execution and management of clinical investigation and post-marketing surveillance with the sponsors and manufacturers. JCROA implements the business activities so as to realize the followings:
- Exploring the ideal CRO in Japan and making efforts for its appropriate establishment, stabilizing and development
- Endeavoring to secure the reliability of quality of contracting services and its results and enhancement, to contribute in efficiency and development of clinical investigations in Japan
- Seek for the ethical and scientific operation of clinical investigations so as to obtain the global evaluation, and to contribute to its internationalization
- Main business activities
- 1. Implementing the best practices of ensuring and improvement of quality and reliability of contracted services
- 2. Driving fair and strict clinical investigation in compliance with regulations
- 3. Executing and searching for the ethical and scientific clinical investigations
- 4. Exploring the way of being of CRO in terms of internationalization of clinical studies
- 5. Executing the public relations to strive the facilitation of clinical investigations and improvement its quality
- 6. Collaboration with regulatory agencies and organizations and providing opinions
- 7. Information sharing contributing to improvement of members as well as conducting education and trainings
|
| Secretariat |
TEL: +81-3-6262-5472
FAX: +81-3-6262-5473
Inquiry by e-mail
URL: http://www.jcroa.or.jp/english/index.html |