Regulatory Process Overview – ACTUAL RESULTS
Numbers of Clinical Trials in Japan
Japan consistently conducts clinical trials for pharmaceuticals, medical devices, and regenerative medicine products. The market is robust, and the clinical trial environment is well-established.
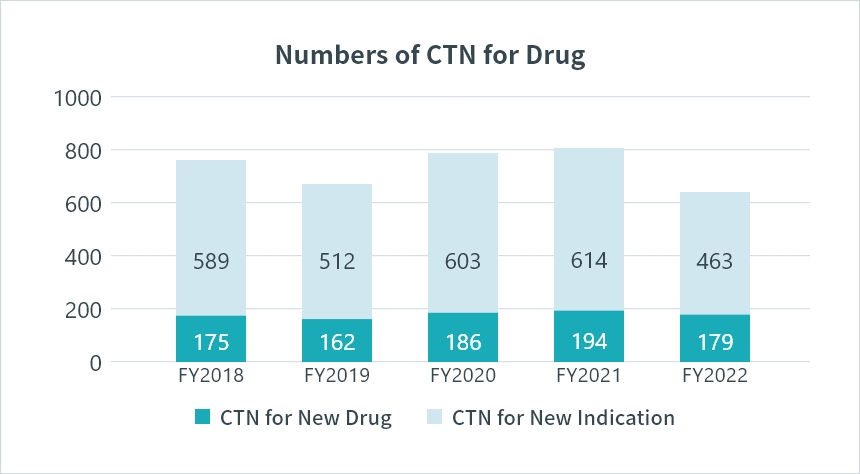
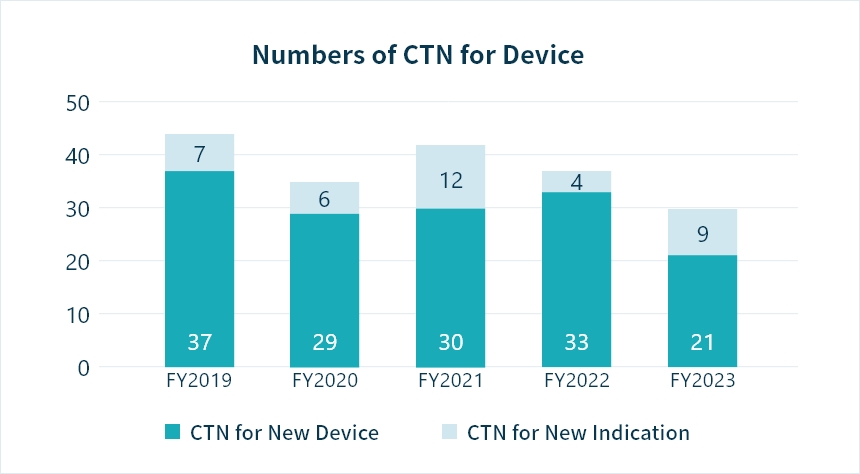
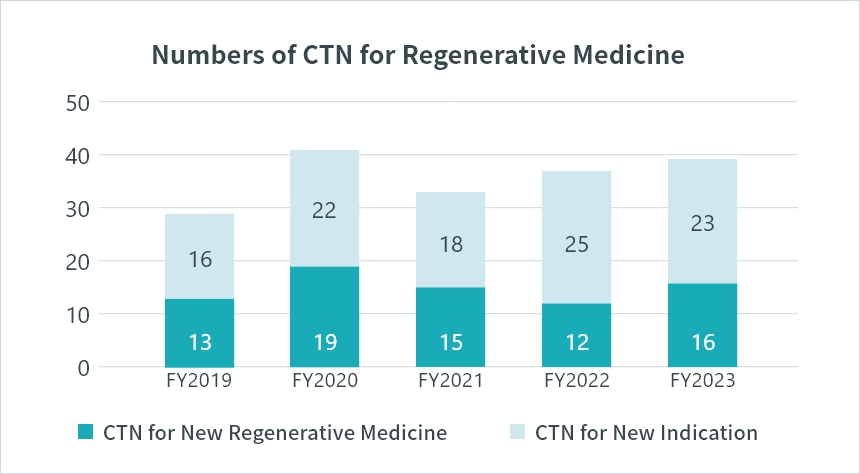
*CTN : Clinical Trial Notification Source: PMDA(https://www.pmda.go.jp/)
Trend in Number Clinical Trial by Trial Type
The number of multi-regional clinical trials (MRCTs) in Japan has been rising annually, with the proportion exceeding 60% since fiscal year 2021. This demonstrates Japan’s well-established environment for conducting MRCTs.
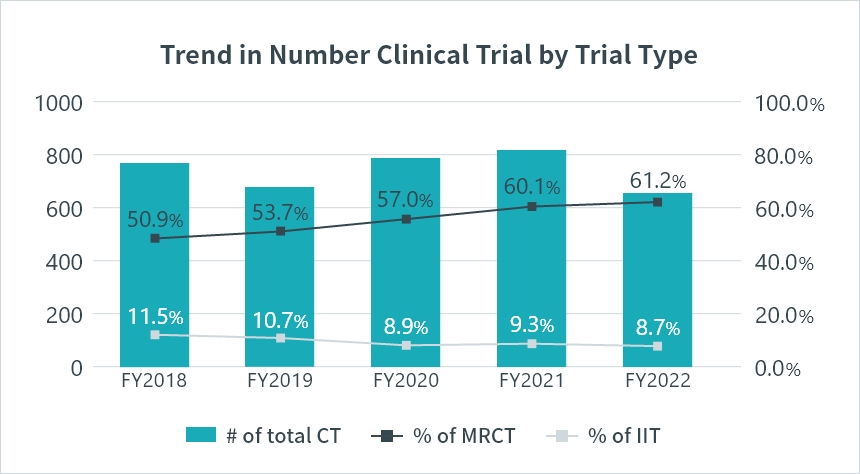
*CT : Clinical Trial
*MRCT : Multi Regional Clinical Trial
Source: PMDA(https://www.pmda.go.jp/)
Numbers of PMDA Consultation
Consultations after Phase II trials have been increasing, indicating that sponsors are actively utilizing PMDA consultations to prepare for the conduct of late-phase clinical trials.
Numbers of Consultation for Drugs and Regenerative Medicines
| Fiscal Year | FY2018 | FY2019 | FY2020 | FY2021 | FY2022 |
|---|---|---|---|---|---|
| Before Phase I Trial Consultation | 41 | 36 | 22 | 31 | 30 |
| Before Phase IIa Trial Consultation | 3 | 10 | 6 | 9 | 5 |
| Before Phase IIb Trial Consultation | 39 | 36 | 39 | 32 | 41 |
| After Phase II Trial Consultation | 154 | 160 | 177 | 185 | 180 |
| Before New Drug Application Consultation | 47 | 62 | 68 | 44 | 55 |
Refer to following link about PMDA Consultation https://www.pmda.go.jp/english/review-services/consultations/0002.html
Source: PMDA(https://www.pmda.go.jp/)
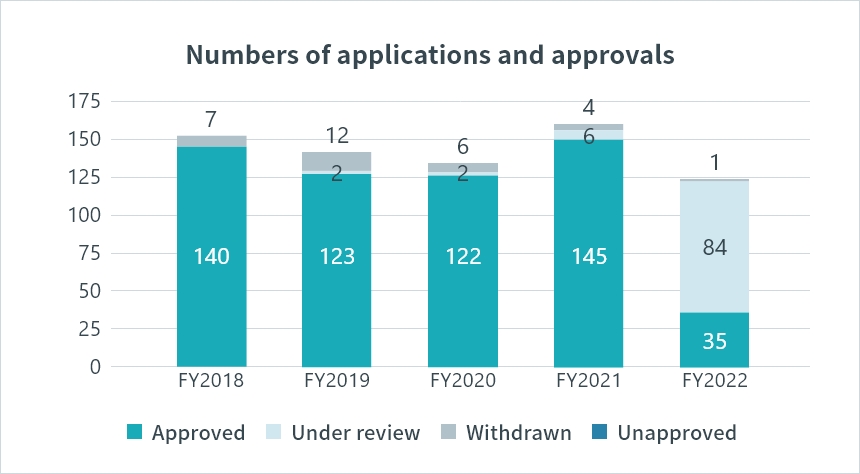
Numbers of New Drug Application (NDA) and Approval in Japan
Over 100 NDA applications are consistently submitted annually, highlighting the PMDA’s robust review system, which guarantees a smooth and timely evaluation process.
Source: PMDA(https://www.pmda.go.jp/)
