Clinical trial industry in Japan
Introduction to Japan’s Clinical Trial Environment
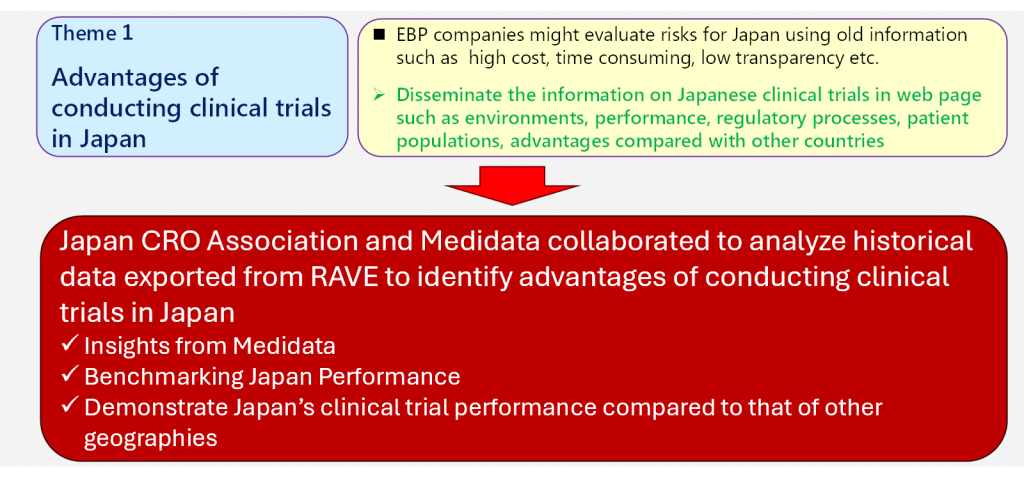
The Japan CRO Association, in collaboration with Medidata, has conducted a comprehensive analysis of Japan’s clinical trial landscape using historical data extracted from Medidata’s Rave platform. This analysis, covering over 2,300 trials across 80 countries, reveals Japan’s significant strengths in data quality and operational reliability within the global clinical research ecosystem.
This document presents these evidence-based findings to global sponsors and stakeholders, aiming to provide a clear understanding of Japan’s current clinical trial environment. By sharing this data-driven perspective, we seek to dispel outdated perceptions and support informed decision-making for international research programs.
We invite you to explore these insights and consider Japan as a strategic hub for your future clinical development activities, leveraging its proven advantages in conducting high-quality, reliable clinical trials.
Advantages of conducting clinical trials in Japan ~analyze historical data~
Advantages of conducting clinical trials in Japan
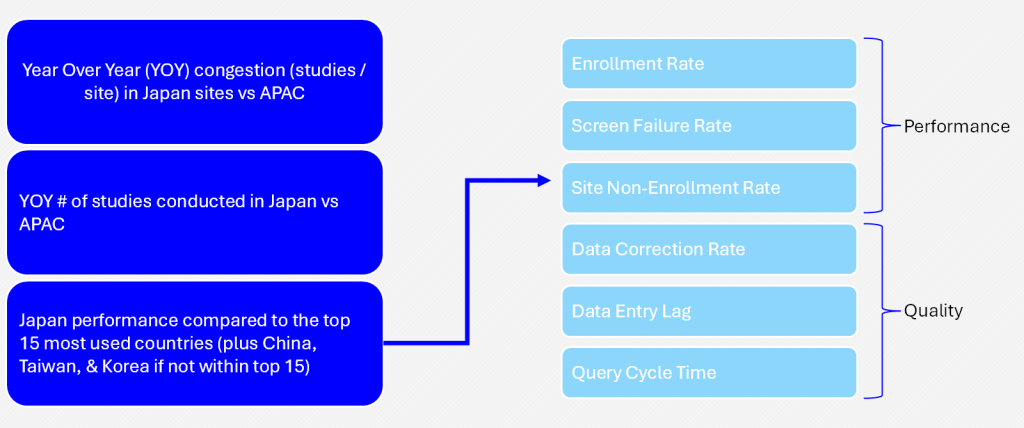
Benchmarks included within analysis
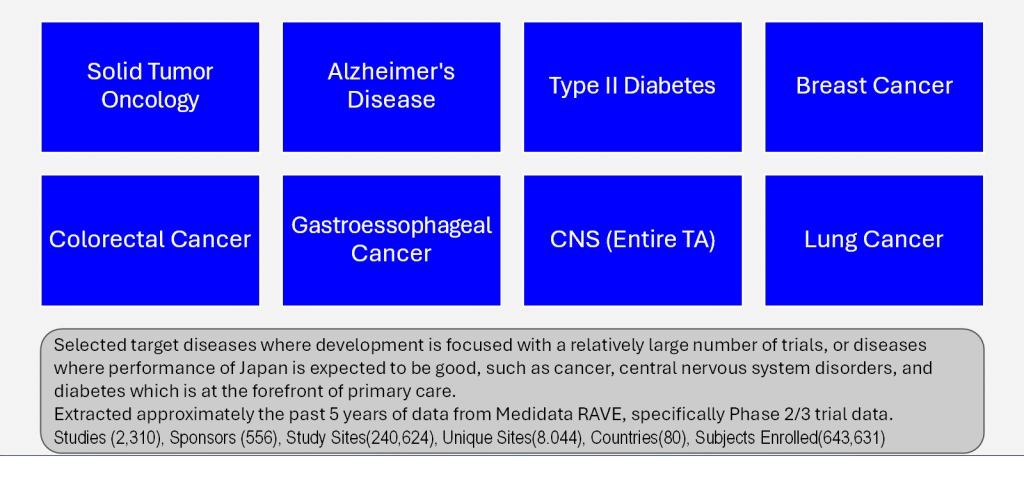
Target diseases
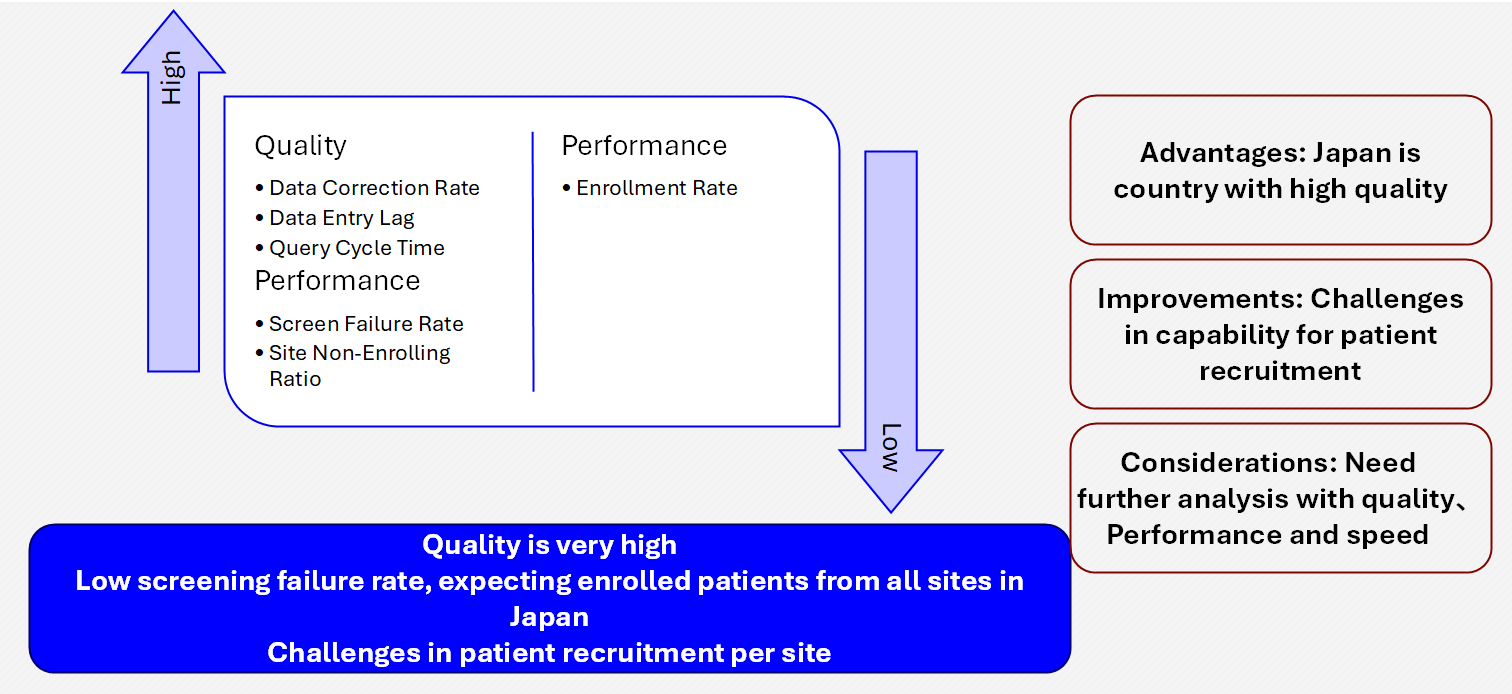
Quality
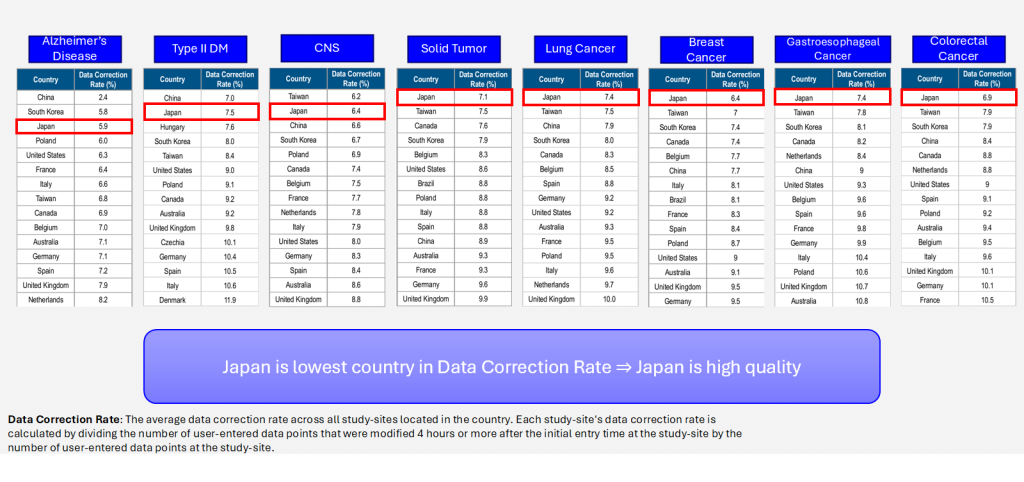
Data Correction Rate
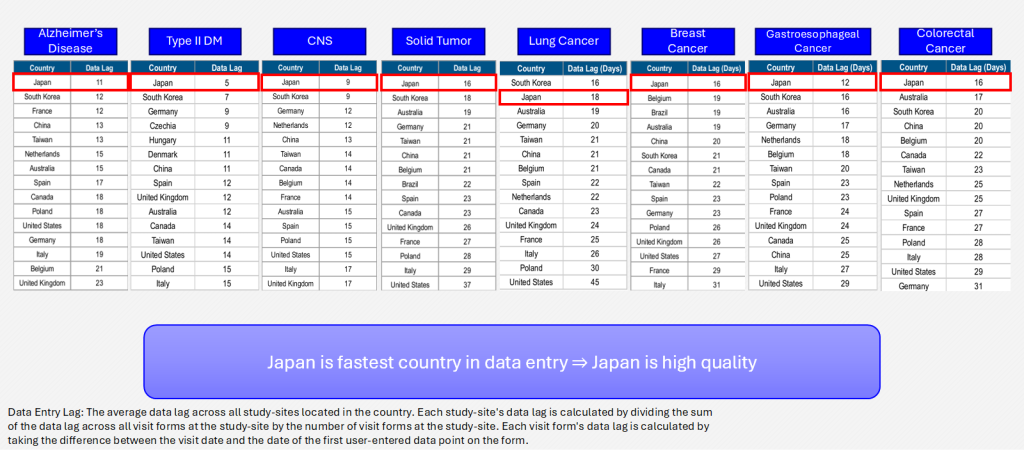
Data Entry Lag
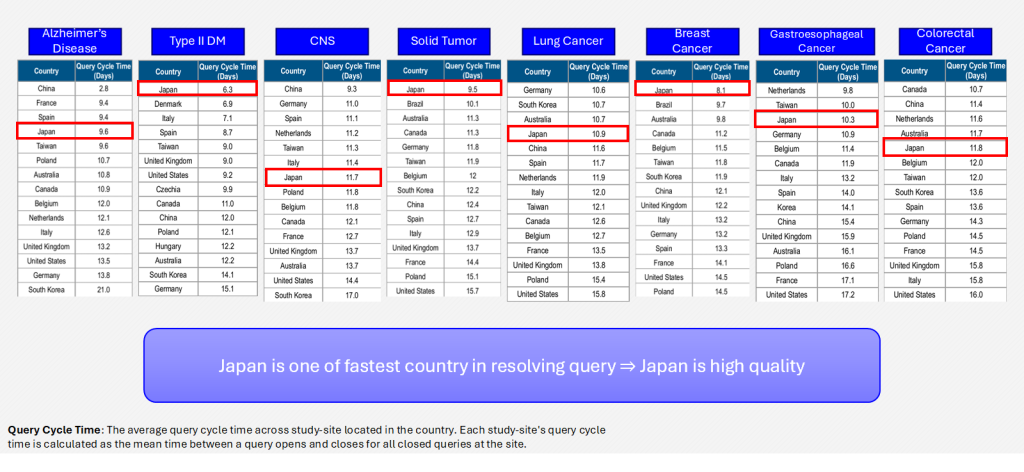
Query Cycle Time
Performance
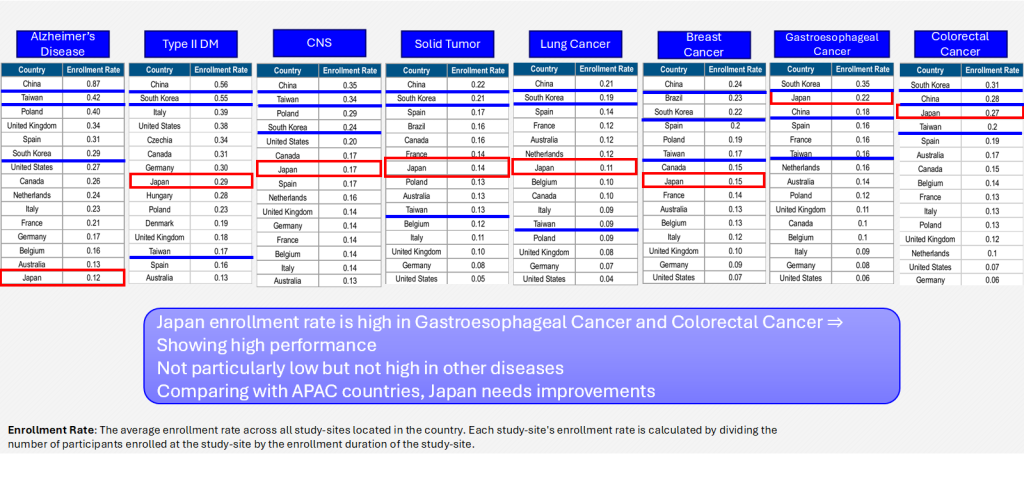
Enrollment Rate per site
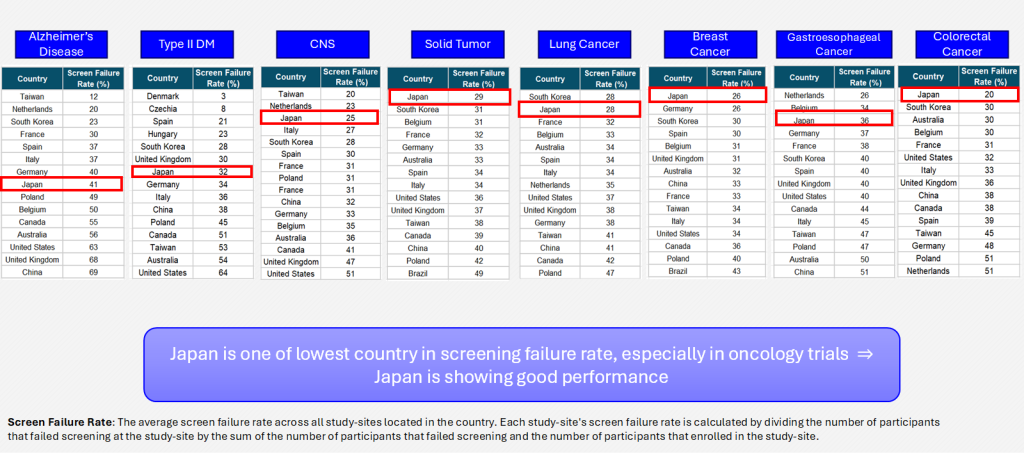
Screen Failure Rate
Non-Enrolling Site Percent
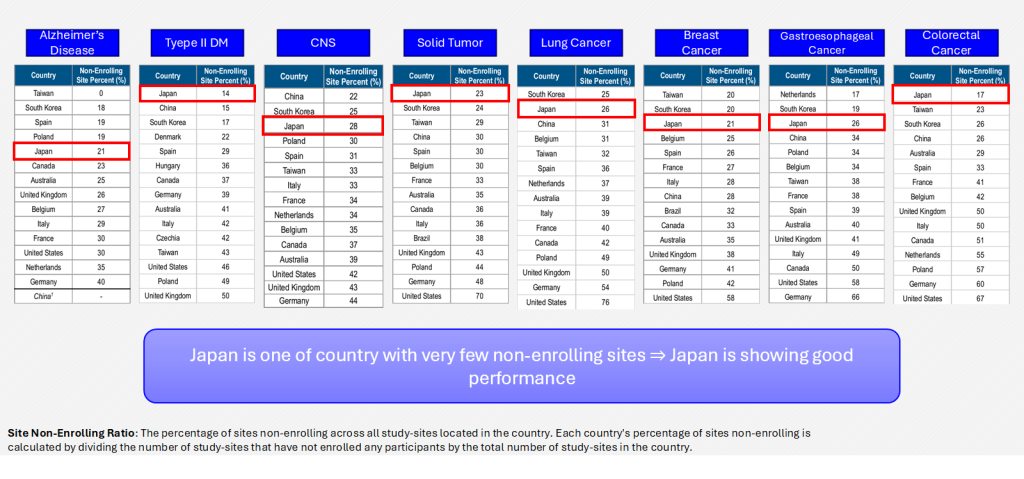
Advantages and improvements identified from historical data